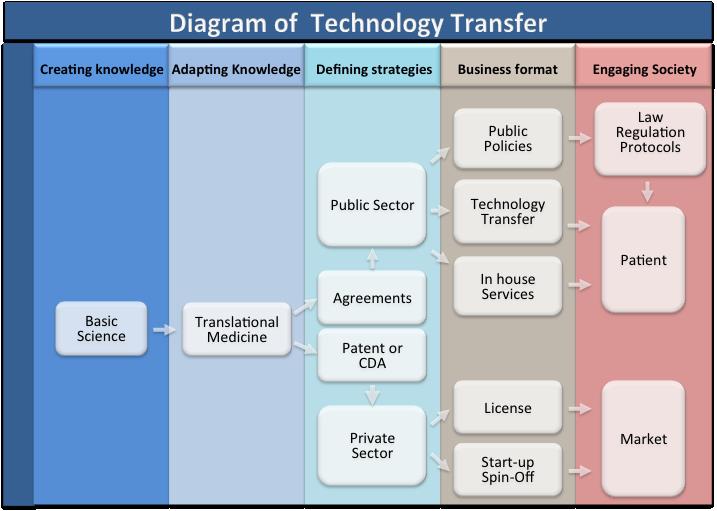
The CTC group has been continuously transferring the results of scientific and technological projects obtained under their supervision to society. We describe here the most promising results obtained in the last two years:
1.1 Prodution of stem or progenitor cells for clinical use;
1.2 Production of recombinant human proteins for the treatment of coagulopathies;
1.3 Development of diagnostics kits and methods to increase the safety of cell therapies;
1.4 Strategies to license our products
During the process of technology transfer to society it became clear that a new product/process generates many challenges for scale-up applications. Therefore, in this report we listthe efforts currently underway not only to create and transfer new technologies, but also to generate packages of solutions that will be required for the technologies to reach society.
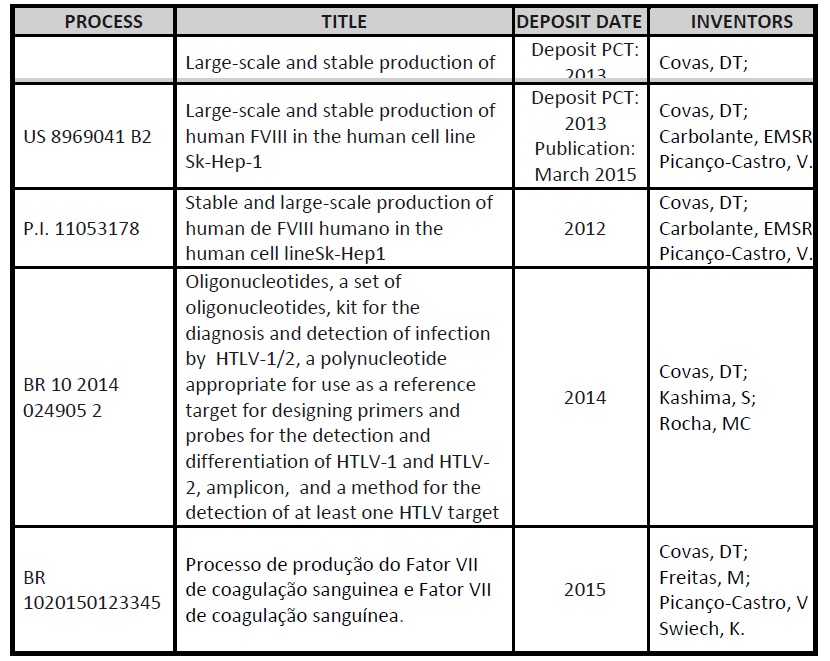
CTC Patents Applications
The team for Technology Transfer Coordination has worked with the USP Agency of Innovation, with SUPERA (Parque de Inovação e Tecnologia de Ribeirão Preto) and also with FIPASE (Fundação Instituto Polo Avançado da Saúde).
1.1. PRODUTION OF STEM OR PROGENITOR CELLS FOR CLINICAL USE
a) Cell-based therapy and cell production under GMP conditions
b) A new xenofree method for cryopreserving MSC
c) Production of human AB serum for the supplementation of culture medium for the cultivation of MSC
d) Large-scale production of MSC
e) Expansion of mesenchymal cells isolated from umbilical cord by the serum-free process
f) Cell transport conveyors: Electronic temperature-controlled conveyor for the transport of somatic stem cells for therapeutic use
1.2 PRODUCTION OF RECOMBINANT HUMAN PROTEINS FOR THE TREATMENT OF COAGULOPATHIES
a) Production of recombinant FVIII (rFVIII)
b) Screening of cell lines for recombinant protein production. (Adaptation of human lines for growth in suspension in FBS-free culture medium)
c) Development of new FVIII molecules that have productive advantages
d) Recombinant FVII
e) Recombinant FIX
f) New method for the quantification of products of biotechnological interest
g) Production of blood factor IX in a bovine bioreactor model
1.3 DEVELOPMENT OF DIAGNOSTICS KITS AND METHODS TO INCREASE THE SAFETY OF CELL THERAPIES
a) Human Cytomegalovirus (HCMV)
b) HTLV – Human T Lymphotropic Virus
c) Human parvovirus B19 (B19V)
d) Human parvovirus 4 (PARV4)
e) Xenotropic murine leukemia virus-related virus ( XMRV)
f) Mycoplasma
g) Chikungunya and Mayaro
1.4 STRATEGIES TO LICENSE OUR PRODUCTS
With the support of the USP Agency, we are taking some actions to obtain the license of patented products, such as: a) a market study to identify clients potentially interested in licensing the inventions, 2) a series of lectures on intellectual property and the importance to obtain a patent. We are going to clarify the legislation and to determine the advantages for the inventor, and 3) presentation of our products/services to selected companies.
Some of these actions are already showing results and we have an ongoing negotiation about one of our products.